GENERAL IN-SERVICE
Part 1: There are New Survey Protocols. Are You Ready?
(Part 1, the Types of Surveys and Level 1 and Level 2 Citations)
CMS has released a revision of the Home Health Agency Survey Protocols and a New State Operations Manual. The new survey process is data-driven and patient outcome-oriented with less structure yet very process-driven. Surveyor worksheets are presently under development and will be released soon by CMS.
The advanced copy of the surveyor procedures introduces a tiered system that directs surveyors to focus on quality of care vs other operations such as HR files. A detailed list of surveyor probes are provided, outlining questions that may be asked throughout the survey process. Agencies should review the questions outlined for surveyors in order to prepare for the survey process. Preparation for this process will reinforce other patient focused processes. Are you ready? To read more, please visit: www.cms.gov/Surveycertificationgeninfo/downloads/SCLetter11_11.pdf
The survey process is guided by interpretive guidelines and survey protocols established to provide guidance for surveyors. They provide clarity as to intent of the regulations. All surveyors are required, by CMS, to utilize these guidelines when evaluating an agency as to compliance with Federal regulation. Remember, the guidelines do not replace regulation and are not allowed to be the basis of any citation, but they provide guidance. Violations are to be based upon clinical record reviews, interviews with patients, caregivers, and personnel, as well as the agency’s practices in relationship to regulation and agency policies.
“The survey and certification process provides a method for CMS to evaluate HHA compliance with the Conditions of Participation (CoPs), ensuring that patient services provided meet minimum health and safety standards and a basic level of quality. The HHA survey process incorporates an approach that is patient-focused, outcome-oriented, and data-driven making it more efficient and effective in assessing, monitoring, and evaluating the quality of care delivered by an HHA…” (Appendix B, p.6).
The surveys are required to have at least one RN on the team. Surveyors are required to attend the HHA Training Course prior to any survey. They are then required to be in an observational role as part of the training.
Types of Surveys
The survey process provides for a standard survey, a partial extended survey, and an extended survey. All HHA must undergo a standard survey.
Initial Certification
The initial certification requires compliance with SS Act1861(0)(4) as well as 2180 regarding licensing requirements. In addition, follow the guidelines of SS2008 “Early Surveys of New Providers and Suppliers.
The State Agency (SA) surveyor or the National Accrediting Organization (AO) inclusive of Joint Commission, CHAP, or ACHC with deeming authority conducts the initial certification. At the time of that survey, the HHA must
- Be operational and have completed the Medicare Enrollment 855A verified by the assigned MAC.
- Provide nursing and one other therapeutic service (42 CFR 484.14(a).
- Meet the new capitalization requirements and have completed an OASIS test submission.
- Have provided care to a minimum of 10 patients requiring SKILLED care.
Part 1: There are New Survey Protocols. Are You Ready? Continuation
Standard Survey
This survey is to be a review of the quality of care and services furnished by the HHA as measured by the medical, nursing, and rehabilitative care indicators. The new changes require this survey to review compliance with regulations most related to high-quality patient care. These highest priority standards (regulations) are called Level 1 standards addressing 9 of the 15CoPs. The thinking is thatif the agency is in compliance with these standards, it is in compliance with all CoPs.
Therefore, “the surveyor can make a determination that the HHA is in compliance with all CoPs when, after a review of the Level 1 standards, and after completing the required clinical record reviews, home visits, and interviews with patients and HHA staff, he/she does not discover any findings which would support a deficiency citation.”
Partial Extended Survey
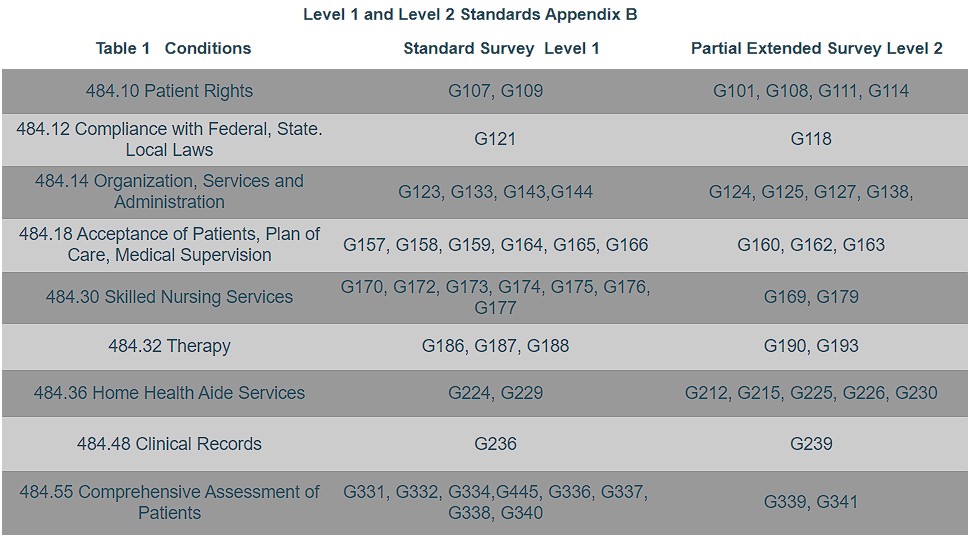
This survey occurs when a standard level survey identifies a non compliant Level I standard and/or a deficiency practice may exist at a standard or conditional level not examined at the standard survey. During this survey, the surveyor reviews at a minimum, the Level 2 standards under the same condition which are related to the non compliant Level 1 standards. See Table 1 Level 1 and Level 2 Standards.
Extended Survey
This survey includes a review of all conditions. It may be conducted at any time at the discretion of CMS and is required to be conducted when any conditional level deficiency is identified. The surveyor is required to review all agency policies, procedures, and practices related to the substandard care (one or more condition –level deficiencies).
Recertification Survey
All HHAs are mandated (SS1891) to have a recertification performed no later than 36 months from a previous recertification survey. These surveys are standard unless a Level 1 citation is leveled.
Now, you know the types of surveys. The following chart lists the standard and partially extended surveys with their related priority standards. The more you know about the new process, the better prepared you will be for your next survey.
Next segment: Surveyors Prep for Survey, Entrance Interviews, Interview Questions They May Ask of Field Personnel and Clinical Managers. Are You Ready?
Part 2: Surveyors Prep for Survey and the New Entrance Interviews
CMS has developed a new survey process for Home Health Agencies that will be effective May 1, 2011. It is data driven, patient outcome-oriented, but according to CMS, is less structured yet very process oriented.
For more detailed information, visit http://www.cms.gov/Surveycertificationgeninfo/downloads/SCLetter11_11.pdfto read the advanced copy.
Under revised survey protocols, agencies will be evaluated on a set of 34 standards, known as Level 1 standards. If the surveyor finds a deficiency on any one of the new highest priority standards, a partial extended survey will be conducted.
During that survey, the agency will be evaluated on 27 Level 2 standards. Both sets of standards fall under the nine conditions of participation. Surveyors must conduct extended surveys of all CoPs when any of the more serious condition level deficiencies are cited. Part 1 of this series outlined each CoP and where the G Tag fell; Level 1 or Level 2.
Many agency leaders are stating that it seems the new survey process has more detailed guidance to reduce surveyor inconsistency.
The survey tasks have been clearly delineated by CMS:
- Task 1- Pre-Survey Preparation
- Task 2- Entrance Interview
- Task 3- Information Gathering
- Task 4- Information Analysis
- Task 5- Exit Conference
- Task 6-Formation of the Statement of Deficiencies
Pre-Survey Preparation
Surveyors will prepare for surveys, more indepthly, using OASIS data, previous survey findings, and complaints filed. Available OASIS reports can be generated for specific time periods, as requested, from the OASIS Coordinator’s office. These reports include case-mix, potentially avoidable events, risk adjusted outcomes based quality improvement (OBQI) or process measure reports.
Part 2: Surveyors Prep for Survey and the New Entrance Interviews Continuation
OBQM Potentially Avoidable Events Report
Know that before coming to the home health agency, the surveyor will have reviewed the most recent quarter of OASIS data to identify patients with emergent care as a result of a fall at home or emergent care for wound infection or deteriorating wound status. This is a Tier 1 event. There are six Tier 2 Potentially Avoidable Events for consideration. To reach the threshold there must be patients who experienced the event and/or the agency to be surveyed must have a current incidence rate equal to or greater than twice the reference rate (Appendix B p.12)
OBQI Outcome Report
Surveyors will also review the agency’s Risk-adjusted Outcomes Report prior to survey. CMS instructs surveyors : “During the onsite survey, select patient records and home visits that focus on the outcomes identified on the OBQI report meeting the individual investigation thresholds” (Appendix B. p12). If none of the ten listed outcomes trigger the selection criteria, another outcome should be selected from the OBQI report (that meets the selection criteria).
Patient/Agency Characteristics Report
As part of the pre survey process, the surveyor will look at this report for the same timeframe as the OBQI Outcome Report and focus on acute conditions and home care diagnoses that are statistically significant or are equal to or greater than 15% points higher than the reference rate. The surveyor is to choose up to three diagnoses or conditions that meet the criteria and look at corresponding patient records.
Error Summary Report by HHA
Surveyors will be looking for several inconsistencies and errors, such as inconsistent M0090 date and incorrect record sequence. The latter error could trigger further record reviews if the HHA’s percent of assessments with this error in or above 10%.
What Can an Agency Do on an Ongoing Basis?
Routinely, agencies should be reviewing the online OASIS reports and identifying areas for improvement. They should show interventions planned and implementation of the plan. The agency should also reflect follow up to implementation. This practice establishes a commitment to Quality Improvement and seeking proactive interventions for areas such as recurring hospital admissions.
Part 3: Entrance Interview
CMS remains detailed as to activities that are to be included in the entrance interview. This interview sets the tone for the survey process identifying expectations.
Part 4: Record Reviews/Home Visits/Analysis/Assigning Citations
CMS has released the new survey protocols, including new guidance as to what HHA surveyors will be expecting from HHA. It is believed the new protocols will provide more survey consistency. According to CMS, the revised survey process incorporated in the protocols is “data-driven, patient outcome-oriented and less structure and process-oriented.” This guidance is effective May 1, 2011.
The protocols focus on the 34 highest-priority standards that closely relate to care quality. During the CMS April 6, 2011 training for surveyors, Pat Sevast (a nurse consultant with the CMS Survey and Certification group) stated that just one finding related to the standards could merit a citation which is a significant move from the present behavior that is seeking non compliant trends at an agency; ie, one of five records or 20% of records reviewed yielded a specific ongoing trend.
With the new survey protocols, a surveyor could cite an agency if just one patient file reflected a patient care issue or a lack of one omitted supervisory visit. Industry leaders expect an increasing number of condition-level citations. The new protocols allow for one standard level citation to trigger a partial extended survey. If that would occur, the agency would be evaluated against the level 2 standards thus increasing their risk for serious citations.
The training for surveyors included Ms Sevast noting that CMS expects surveyors to cite at a condition level the patient rights’ conditions of participation (CoP) if an agency is out of compliance with two of the highest-priority standards and one level 2 violation. That would trigger an automatic extended survey necessitating review of all CoPs.
So what should an agency do?
Agencies should review the new survey protocols and become familiar with the Home Health “G” Tags and Abbreviated Identifiers, HHA Survey Investigation Worksheets and Calendar, and HHA Survey Investigation Worksheets as well as the Revised Home Health Survey Protocols of February 11, 2011 and the advanced copy of Appendix B- Guidance to Surveyors.
Parts 1-3 of the Select Data article regarding Survey Protocols published in the March 30, 2011 ezine looked at the types of surveys, level 1 and 2 citations, surveyor prep for the survey as well as the new entrance interviews, and the entrance information with specific information gathering techniques.
Part 4: Record Reviews/Home Visits/Analysis/Assigning Citations Continuation
This segment looks at the clinical records and home visits.
The number of records reviewed is still determined by the unduplicated census of the prior year as well as the number of records and home visits necessary to assess compliance with the CoPs. There is an increase in required home visits by the surveyor as the focus is essentially patient care oriented.
Home visits to patients should include those receiving high-tech care, home health aide services as well as patients triggering “at risk” of Level 1 and Level 2 potentially avoidable events. Some of the areas the surveyor will be looking at:
- storage of records,
- the most recent plan of care and its specificity as to orders and goals,
- when the patient was visited in relation to the physician’s order,
- completeness of the comprehensive assessment,
- evidence of “major decline or improvement,”
- how coordination of services are met,
- any evidence of the patient/caregiver contributing,
- care provisions not in compliance with the law,
- case conferences, informal conferences and telephone calls,
- patient specificity of the plans and visits,
- evidence of patients denied or not offered services,
- patients hospitalized,
- patients with LUPAs,
- reconciliation of care provided to orders given by the physician,
- inter-related factors of patients with co-morbidities and the care received,
- therapy visits made at ordered frequency,
- evidence that PTAs, COTAs, and LVN/LPNs were supervised appropriately,
- evidence home health aide visits were made every two weeks,
- if an RN or PT ever observed the aide’s provision of care,
- evidence the aide careplan was specific to the patient,
- evidence of consistent documentation of VS, insulin injections, B/P, pain frequency/ severity/interventions,
- how corrections are made in the record,
- evidence of discharge summaries in discharge records,
- evidence of consistent assessment of patient status and progress over the visits.
Part 4: Record Reviews/Home Visits/Analysis/Assigning Citations Continuation
The home visit and interviews.
Home visit probes will focus on “compliance related to patient rights, accepted professional standards of practice, coordination of care, and comprehensive assessment of patients, plan of care, services provided, and clinical records.” Though not all inclusive, consider the surveyor will be looking at:
- any instances of personnel providing care that may not be in accordance with laws, regulations, state practice acts, accepted professional standards, or agency policies and procedures,
- communication by providers with patients/caregivers,
- evidence that care is delivered by accepted professional standards,
- evidence that care providers follow CDC guidelines,
- evidence the aide follows the plan as identified by written instructions,
- evidence that “medications in the home are the same as those listed on plan of care, interim orders, and clinical record notes,”
- and asking the clinical personnel “about instances of patient care noted in home visits or record reviews that deviated from the physician orders, accepted professional standards or agency policy.”
The surveyor will interview the patient caregiver to validate that care documented in the plan is the care that is provided, will ascertain if needs are being met by the agency, identify if caregivers are satisfied with the care, that medications presently taken are what have been prescribed (and will compare it to physician orders found in the clinical record), that there is participation by the patient/caregiver in the planning of care, and if they understand the process for handling a complaint. These are minimum areas of review and the agency should be aware that the surveyor may ask when visits occurred, did the clinician and care provider wash their hands, and did they bring their own towels? The surveyor may ask to see all medications taken, including OTC meds and engage the patient/caregiver in discussing when and how they take the meds.
It is important that agencies review processes that are in place to be certain that appropriate agency personnel understand policy and those procedures that support that policy. There needs to be consistency of statements when speaking with the surveyors, who will now have a greater number of interviews scheduled then documented.
Part 4: Record Reviews/Home Visits/Analysis/Assigning Citations Continuation
The information analysis
This process requires surveyors to review the information gathered during the survey and exercise judgments about the effect of care upon patient outcomes, the degree of severity of any behaviors not fully in compliance, the frequency of the non compliance, and how the services were impacted.
Standard and Condition Level Deficiencies
Data Tags (G-Tags) are assigned to the standards in the interpretive guidelines. If a data tag is assigned to a condition it becomes a condition level data tag. If assigned to a standard level deficiency it is cited at a standard level tag.
If a Level 1 standard-level deficiency is identified, “the surveyor is required to move to a partial extended survey and the surveyor examines, at a minimum, the Level 2 standards under the same condition and any other standards the surveyor chooses to examine.” A review of all Level 2 standards that relate to a deficiency at Level 1 standards is the minimum requirement.
Any condition level deficiency “requires a move to an extended survey which includes a review of all CoPs and the policies and procedures that resulted in the substandard care.” Substandard care is defined by CMS as “one or more CoPs out of compliance.”
Summary
The new survey process is data-driven and begins with the surveyor’s pre-survey preparation. The surveyor will be focusing on patient care and outcomes derived. The Appendix B of the State Operations Manual has been revised and all are encouraged to read about the new survey process. The definition of a standard survey has been revised to increase the survey’s focus on those standards most related to patient care. Surveyor worksheets are available online at the CMS worksite and provide insight as to the depth and path of the survey. CMS has established a special mailbox for questions related to the new survey protocols hhasurveyprotocols@cms.hhs.gov. Appendix B Guidance to Surveyors: Home Health Agencies of the State Operations Manual offers, in addition to the surveyor process, a full listing of the G-Tags and the interpretive guidelines allowing the agency to see the basis for the interview questions.
This survey process is believed to offer more consistency and focus. The new process complements the patient/outcome focus of OASIS and the drive for improved outcomes and quality patient care. The surveyors training has been completed. It would be interesting to hear from agencies that experience the new process. Arm yourself with information.
Video
How the training works
The training focuses on prevention of surgical site infections, central lines associated bloodstream infections, ventilator-associated pneumonia, Catheter-associated urinary tract infections, Clostridium difficile and Methicillin-resistant Staphylococcus aureus (MRSA). In addition, it includes information on basic protocols for universal precautions and isolation precautions to protect patients, visitors, and practitioners from the most common disease transmissions. The training promotes these key behaviors:
• Teamwork
• Communication
• Hand washing
• Vaccination against the flu
• Appropriate use of antibiotics
• Proper insertion, use, and removal of catheters and ventilators
Trainees assume the identity of characters in a computer-based video simulation and make decisions as each of those characters. Based upon their decisions, the training branches to different pathways and patient outcomes. The training is designed and developed for use by groups in facilitated training sessions and by individuals as a self-paced learning tool.
LINKS
Please click the links below to review the topics we offer.
Safety Primers - This will guide you through key concepts in patient safety. Each primer defines a topic, offers background information on its epidemiology and context, and highlights relevant content from both AHRQ PSNet and AHRQ WebM&M.


